Fill a Valid Negative Hiv Test Form
The Negative HIV Test form serves as a vital document in the process of HIV testing, ensuring that clients receive accurate and timely information regarding their health status. This form includes essential client information such as name, date of birth, and race, alongside details about the testing location. It clearly indicates the results of the HIV antibody screening test, categorizing them as either reactive or negative/non-reactive. Additionally, the form outlines the importance of follow-up appointments, providing space for scheduling further consultations if necessary. To maintain quality control, the form also features temperature logs for the storage of rapid HIV test devices and controls, ensuring that tests are conducted under optimal conditions. Each section of the form requires signatures from both the client and the counselor, reinforcing accountability and transparency in the testing process. By systematically documenting these elements, the Negative HIV Test form plays a crucial role in promoting public health and supporting individuals in managing their HIV status effectively.
Additional PDF Templates
Passport Form Ds-82 - Researching additional requirements for international travel can be beneficial prior to applying.
Planned Parenthood Pregnancy Verification Letter - Monthly income and family size are requested to assess eligibility for services.
Understanding the importance of having a formal record during the sale process is key to avoiding any disputes in the future. The Georgia RV Bill of Sale form can be easily accessed online, ensuring that all necessary information is captured correctly. For a seamless experience, you can find the form at https://georgiapdf.com/rv-bill-of-sale, which will guide you through the steps needed to complete the sale.
What Is a P45 in the Uk - Employees must remember to provide the P45 to their new employer before starting work.
Similar forms
- Medical Test Result Form: Similar to the Negative HIV Test form, this document records the results of various medical tests, including patient details, test type, and outcomes. Both forms require signatures from the client and the healthcare provider.
- Consent Form: This document is used to obtain permission from clients before conducting tests. Like the Negative HIV Test form, it includes client information and ensures that the client understands the procedure and its implications.
- Patient Intake Form: This form collects essential information about a patient before testing. It shares similarities with the Negative HIV Test form in that it gathers demographic data and medical history to inform the testing process.
- Follow-Up Appointment Confirmation: This document schedules and confirms follow-up visits after testing. It parallels the Negative HIV Test form by including details about the appointment and requiring client acknowledgment.
-
New York ATV Bill of Sale: This form is critical for documenting the sale of an ATV, ensuring both buyer and seller are protected during the transaction. For further assistance and resources, you can visit freebusinessforms.org.
- Laboratory Chain of Custody Form: This document tracks the handling of specimens in a lab setting. It is similar in that it ensures accurate documentation of the testing process and maintains the integrity of the results.
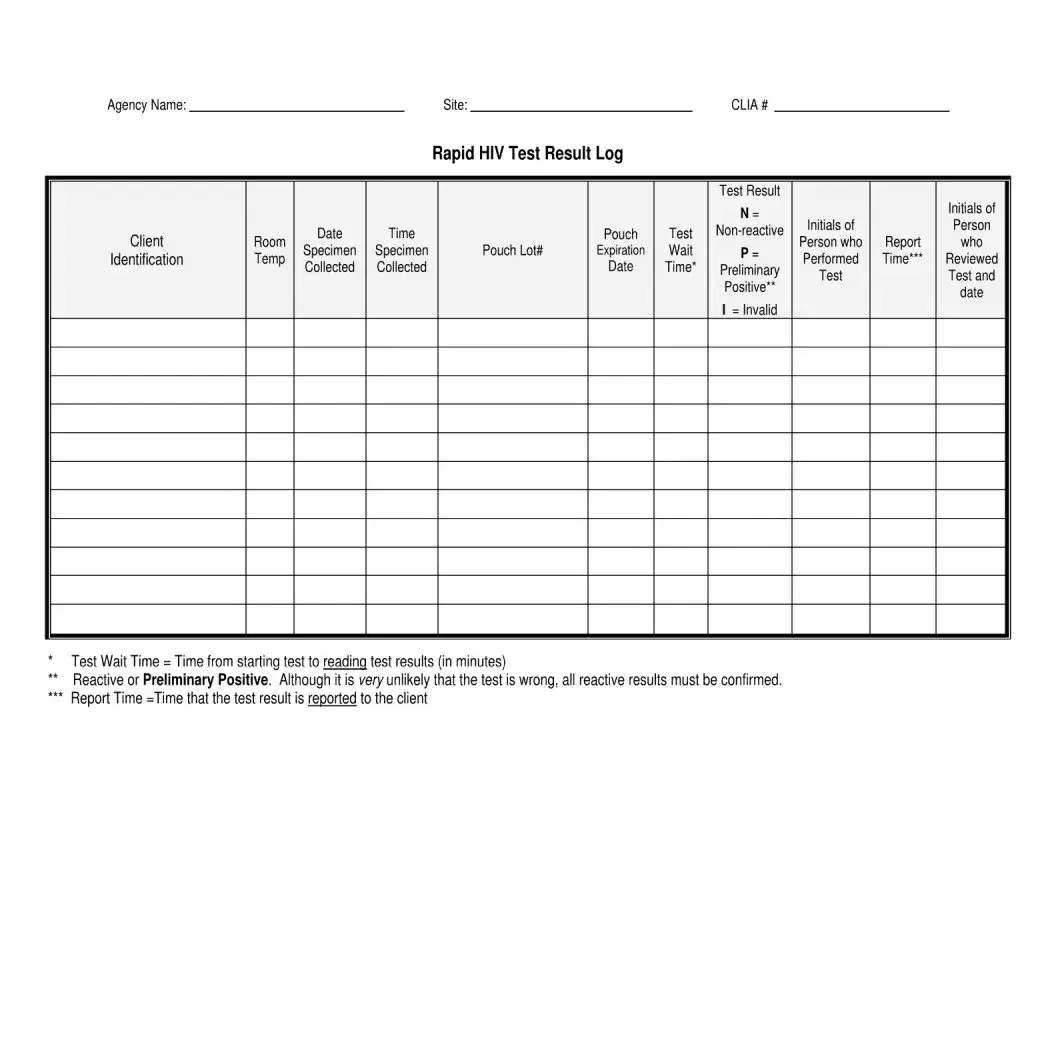
- Test Result Log: This log records all test results conducted at a facility. Much like the Negative HIV Test form, it includes details such as client identification and test outcomes, ensuring proper tracking and reporting.
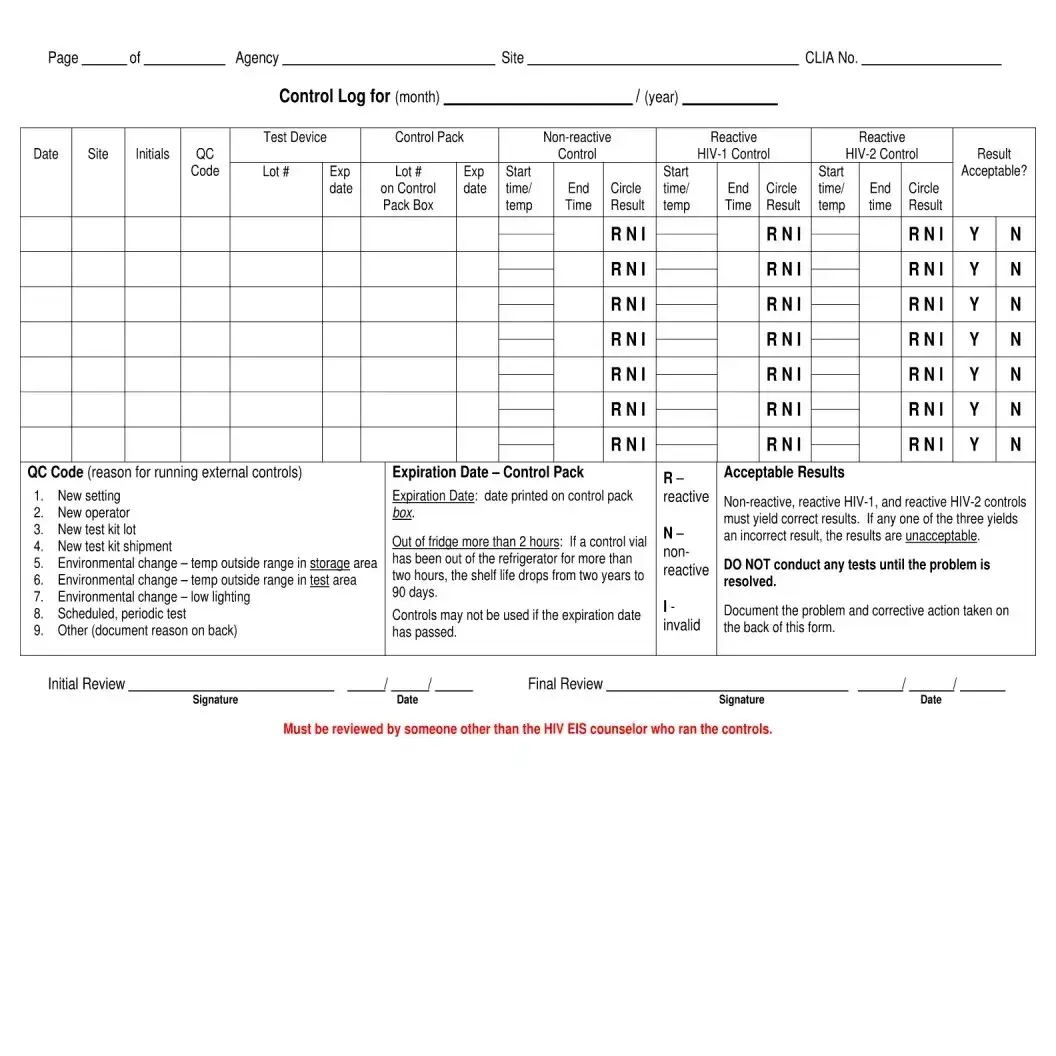
- Quality Control Log: This document monitors the performance of testing equipment. It shares characteristics with the Negative HIV Test form by documenting essential data, ensuring that tests are reliable and accurate.
Document Example






Form Specs
| Fact Name | Description |
|---|---|
| Client Information | The form requires the client's name, date of birth, sex, and race to ensure proper identification and demographic tracking. |
| Testing Result | The HIV Antibody Screening Test Result section indicates whether the result is reactive or negative/non-reactive, which is crucial for follow-up actions. |
| Follow-Up Appointment | A section for documenting the follow-up appointment details is included, ensuring that clients receive necessary post-test support. |
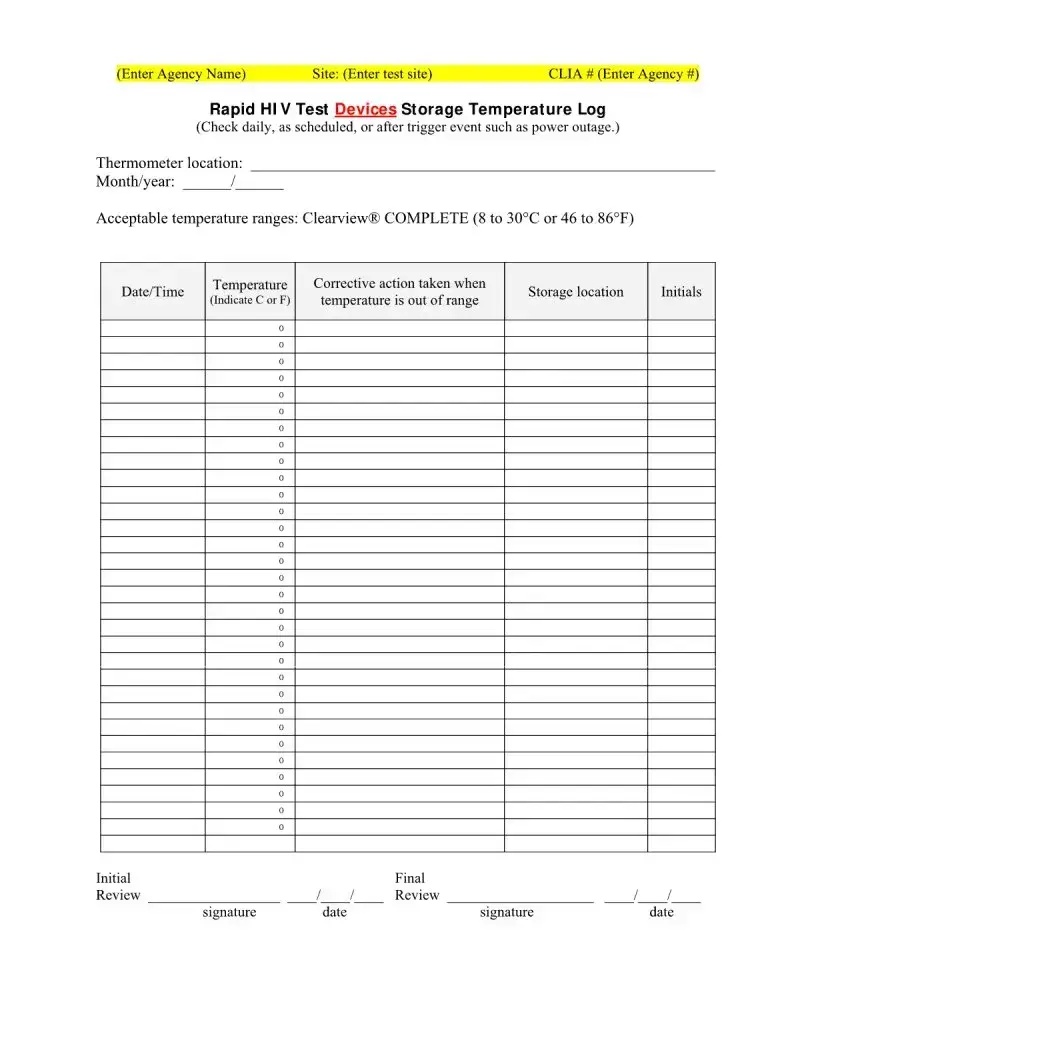
| Storage Temperature Log | The form includes a temperature log for storing test devices and controls, ensuring compliance with storage requirements for accurate test results. |
| Governing Laws | State-specific forms must comply with local health department regulations and CLIA (Clinical Laboratory Improvement Amendments) requirements. |
Crucial Questions on This Form
What is the purpose of the Negative HIV Test form?
The Negative HIV Test form is used to document the results of an HIV antibody screening test. It provides essential information about the client, including their name, date of birth, and testing location. This form serves as an official record that the client has received a negative or non-reactive result, which is crucial for their health management.
What information is included in the form?
The form includes the following details:
- Client Name
- Date of Birth
- Date of the test
- Sex
- Race
- Testing Location
- HIV Antibody Screening Test Result (Reactive or Negative/Non-Reactive)
- Follow-Up Appointment details
- Signatures of the client and counselor
What does a negative result mean?
A negative result indicates that no HIV antibodies were detected in the client's blood at the time of testing. This generally means that the client is not infected with HIV. However, it is essential to consider the timing of the test, as it may not detect recent infections. If there is a risk of exposure, follow-up testing may be recommended.
How is the test conducted?
The test is typically conducted using a rapid HIV test device. A healthcare professional collects a sample, often through a finger prick or a blood draw, and then processes it according to the manufacturer's instructions. Results are usually available within minutes.
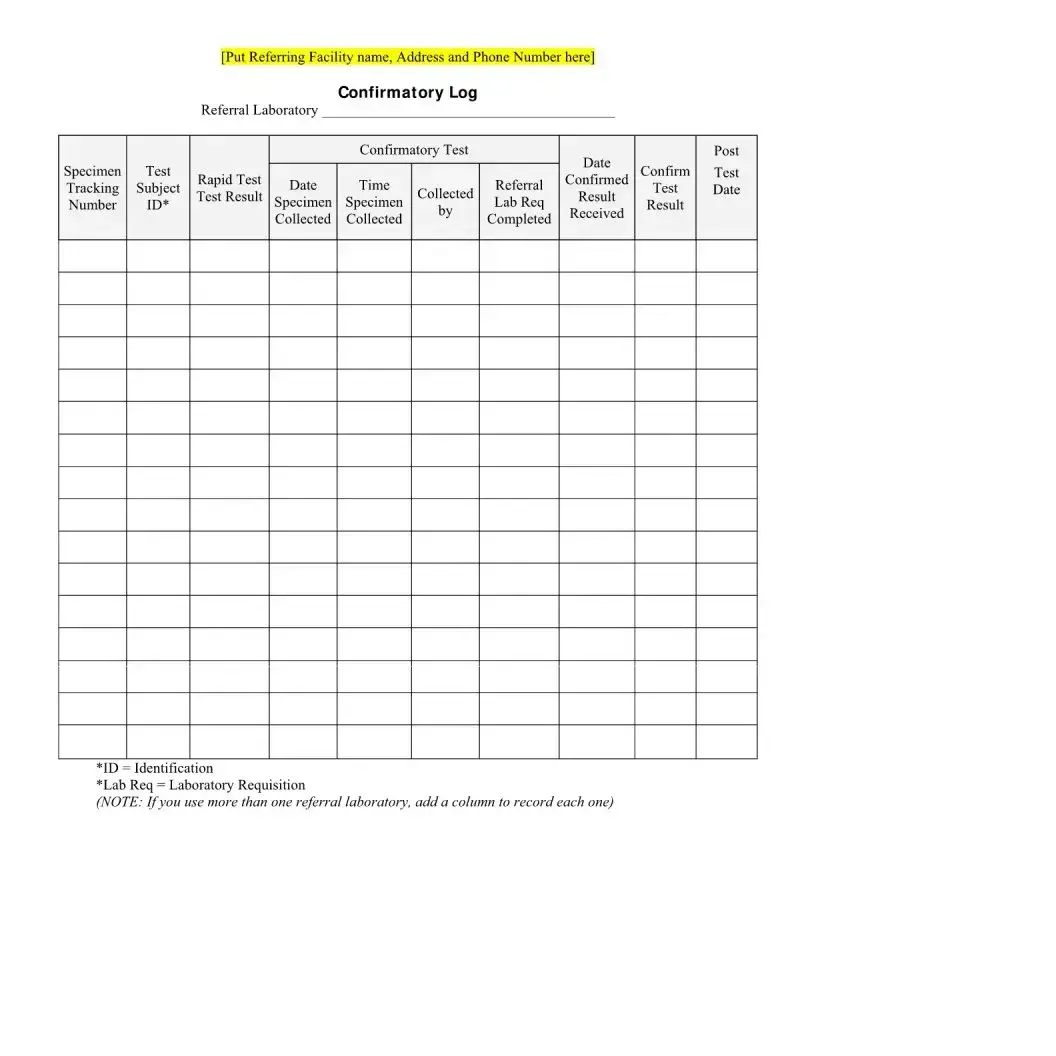
What should I do if I receive a reactive result?
If a client receives a reactive result, it is crucial to seek confirmation through additional testing. Reactive results are not definitive for HIV infection; they require further evaluation. A healthcare provider will guide the client on the next steps, including confirmatory tests.
What is the importance of follow-up appointments?
Follow-up appointments are essential for clients who receive a reactive result or those who have concerns about their HIV status. These appointments allow healthcare providers to discuss the results, provide counseling, and recommend further testing or treatment options if necessary.
Who can access my test results?
Test results are confidential and should only be shared with the client and authorized healthcare providers. The form may include signatures to ensure that the client understands and consents to the sharing of their results, maintaining their privacy and confidentiality.
What are the acceptable storage temperatures for the test devices?
The acceptable storage temperature for the Clearview® COMPLETE HIV test devices is between 8 to 30°C (46 to 86°F). It is essential to monitor and document storage temperatures regularly to ensure the accuracy and reliability of the test results.
What happens if the storage temperature is out of range?
If the storage temperature of the test devices is found to be out of the acceptable range, corrective actions must be taken immediately. This may include relocating the devices to a suitable environment and documenting the incident in the storage temperature log.
Documents used along the form
When dealing with a Negative HIV Test form, several other documents often accompany it to ensure comprehensive patient care and accurate record-keeping. These documents serve various purposes, from tracking test results to maintaining proper storage conditions for testing materials. Below is a list of related forms that are commonly used in conjunction with the Negative HIV Test form.
- Client Consent Form: This document ensures that the client has given informed consent for testing. It outlines the purpose of the test, potential risks, and the client's rights regarding confidentiality.
- Test Result Notification Form: This form is used to formally notify clients of their test results. It includes details about the result, follow-up actions, and any necessary referrals for additional care.
- Follow-Up Appointment Schedule: This document records the details of any follow-up appointments after testing. It specifies the date, time, location, and purpose of the follow-up visit.
- Quality Control Log: This log tracks the quality control measures taken during testing. It ensures that testing procedures meet established standards and that any issues are documented and addressed.
- Temperature Log for Test Storage: This log monitors the storage conditions of testing materials. It records daily temperatures to ensure that they remain within acceptable ranges, which is crucial for test accuracy.
- Living Will Form: This crucial document outlines an individual's medical treatment preferences when they are unable to decide for themselves, ensuring their choices are respected. For more information, visit All Ohio Forms.
- Test Result Log: This document maintains a record of all test results. It includes client identifiers, test dates, results, and the initials of personnel involved in the testing process, ensuring accountability and traceability.
These forms and documents play a vital role in the testing process, ensuring that clients receive accurate information and appropriate follow-up care. Proper management of these documents contributes to the overall effectiveness of HIV testing and treatment programs.
Misconceptions
- Misconception 1: A negative HIV test means you are free of HIV for life.
- Misconception 2: You can rely solely on a negative result from a rapid HIV test.
- Misconception 3: All negative results are definitive and do not require further action.
- Misconception 4: HIV testing is only necessary if you have symptoms.
- Misconception 5: A negative test means your partner is also HIV negative.
- Misconception 6: You can stop using protection if you receive a negative test result.
- Misconception 7: All HIV tests are the same and provide the same level of accuracy.
- Misconception 8: A negative result means you cannot transmit HIV.
A negative result only reflects your status at the time of testing. If you engage in high-risk behaviors after the test, you could still contract HIV.
While rapid tests are accurate, they may not detect recent infections. Follow-up testing is essential for confirmation.
Negative results should be interpreted in the context of your risk factors and testing history. Regular testing is recommended for those at risk.
Many people with HIV do not show symptoms for years. Routine testing is crucial for early detection and treatment.
Your test results do not reflect your partner's status. Both partners should get tested to ensure safety.
Using protection remains important, even with a negative result. It reduces the risk of HIV and other sexually transmitted infections.
Different tests have varying levels of sensitivity and specificity. Understanding the type of test used is important for interpreting results.
A negative result only indicates your status at the time of testing. If you are exposed to HIV after the test, you can still transmit the virus.
